Pharmaceutical and Vaccine Quality Illustrated is now available for free download and online use

“Pharmaceutical and Vaccine Quality Illustrated” is now available for free download (PDF version) and for online use in EPELA platform.
The work includes 730 terms, 101 photographs, 77 illustrations, 51 tables, 191 references, links and QR codes to 26 videos and 4 gif files in 255 pages. The work is more than a glossary, it also includes tools and approaches applicable alongside with the definitions. The EPUB version will be coming soon.
The spectrum of this glossary goes from product development and clinical trials to legislation around regulatory functions; from manufacturing to storage and dispatch activities and to post-marketing surveillance. It covers a comprehensive list of systems, procedures, and tools used at all levels that one way or another touch the issue of product quality. What you have in your hand is not a simple glossary. Under many terms, in addition to their definitions, you will find additional information and perspectives on their uses. Wherever possible, tables, flow charts, decision tables, illustrations, sketches, and photographs are included.
EPELA authentic e-Pharmaceutical Cold Chain Management course receives 2015 Hermes Creative Award as Gold Winner in e-learning category

On 1 May 2015, Hermes Creative Awards announced winners for the 2015 international awards competition for creative professionals involved in the concept, writing and design of traditional and emerging media. Hermes Creative Awards recognizes outstanding work in the industry while promoting the philanthropic nature of marketing and communication professionals. EPELA authentic e-Pharmaceutical Cold Chain Management course was announced as the Gold Winner in e-Learning category.

There were over 6,000 entries from throughout the United States, Canada and several other countries in the Hermes Creative Awards 2015 competition. Entries came from corporate marketing and communication departments, advertising agencies, PR firms, design shops, production companies and freelancers.
Hermes Creative Awards is administered and judged by the Association of Marketing and Communication Professionals (AMCP). The international organization consists of several thousand marketing, communication, advertising, public relations, media production and free-lance professionals. AMCP oversees awards and recognition programs, provides judges and rewards outstanding achievement and service to the profession.
As part of its mission, AMCP fosters and supports the efforts of creative professionals who contribute their unique talents to public service and charitable organizations. Hermes entrants are not charged entry fees to enter work they produced pro bono. In addition, the efforts of generous marketing and communication professionals are acknowledged through grants and special recognition.
AMCP judges are industry professionals who look for companies and individuals whose talent exceeds a high standard of excellence and whose work serves as a benchmark for the industry. Winners were selected from 195 categories grouped under advertising, publications, marketing/branding, integrated marketing, public relations/communications, and electronic media and pro bono.
A list of Platinum and Gold Winners can be found on the Hermes Creative Awards website (https://enter.hermesawards.com/winners/#/gold/2015)
On 22 February 2015, we lost Andrew Garnett... A dear friend, mentor, and an irreplaceable brain… A huge loss to the world of immunization.

I met Andrew in 2001 during the revision of international packaging and shipping guidelines. From that day on, we became close friends and have worked on numerous projects. It is with Andrew that we brought to life the Effective Vaccine Store Management (EVSM) initiative and Performance, Quality and Safety (PQS) project.
Andrew was the number one choice as an author on cold chain management guidelines. He was an immaculate editor for many technical series we worked on together.
As an architect, Andrew was THE master of mind-maps. He fascinated me with his ability to bring about a clear mind-map structure to any complex concept.
Andrew was one of the four mentors teaching in EPELA e-Pharmaceutical Cold Chain management course. His insightful reviews and feedback to participants were deeply appreciated and will always be remembered.
I deeply admired you, Andrew, for your elegance, your subtle artistic taste, your scientific rigor, your passion for life…
I shall miss you dearly, Andrew.
Umit Kartoglu
EPELA integrates Flipgrid to VVM and cold chain authentic e-learning courses
Flipgrid, developed by the LT Media Lab at the University of Minnesota, is a web-based platform (for asynchronous communication) that allows to create “grids” of discussion-style questions for participants to record their views with a video message that is limited to 90 seconds. All the video responses end up in a grid which makes it visually attractive and very easy to listen to what all other colleagues have to say.
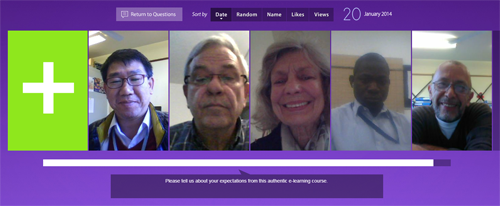
The integration of Flipgrid will be done through a design-based research project developed jointly by EPELA and LT Media Lab at the University of Minnesota. Through this design-based research, we will be able to address complex problems in real context in collaboration with practitioners. It will also allow us to integrate known and hypothetical design-principles with technological affordances to render plausible solutions to these complex problems. We will be conducting rigorous and reflective inquiry to test and refine integration and use of Flipgrid as an innovative learning environment.
Flipgrid will be used for introductory activities prior to the course as well as for discussion, diary, evaluation during the course, and end-course feed-back and post-course support.
EPELA launches its new authentic e-learning course on VVM based vaccine management.
EPELA launches its new authentic e-learning course on VVM based vaccine management.
The 9-week course takes participants through a sophisticated vaccine chain to make the best use of VVM through ensuring that the vaccines have not been damaged by heat, reducing vaccine wastage, pinpointing cold chain problems, managing stocks, facilitating immunization outreach and increasing access and improving vaccination coverage, preventing inadvertent freezing, and providing hints whether an opened multi-dose vaccine vial may be used in a subsequent immunization session.
Tools are offered to work in groups, evaluation is done through self, peer and expert reviews. Nobody lectures any participants in this course, but we make expert videos and critical documents available to participants through video and document libraries.
The course will be mentored by Umit Kartoglu, Julie Milstien, Ticky Raubenheimer and Denis Maire.
The new course will run as beta-course for the first time between 27 January and 28 March 2014. Regular courses are scheduled to take place during the following periods:
- 21 April - 20 June 2014
- 6 October - 5 December 2014
For details of the course please check at http://epela.net/epela_web/evvm.html
Getting ready for the VVM course’ kick-off
EPELA design team met in Istanbul in September 2011 to discuss the development of the e-course on the VVM-based vaccine management. Ümit Kartoğlu (course director), Gokhan Gurses (creative director) and Ted Prusik (sponsor) reviewed the major issues for the content development and laid out the timeline to bring the course to life in early 2013. In addition to the e-course, a learning/educational package will be prepared and oriented to the needs of vaccine manufacturers. Video shootings for the development of vaccine manufacturers package were completed in Temptime’s New Jersey facilities and at BioFarma in Indonesia in June 2012. The team (Umit Kivanc, cinematographer; Gencer Yurttas, camera assistant; and Umit Kartoglu, course director) also completed some case studies for the illustrated lectures while in Indonesia. Illustrated lectures were shot in Istanbul. More case studies have also been shot in Madagascar in January 2013. The course will be facilitated by a group of experienced mentors, who are well-known in the field of the VVM-based vaccine management. It is expected that the VVM course will be offered to the professional audience in 2013.
NEWS
Pharmaceutical and
 Vaccine Quality Illustrated is now available for free download and online use
Vaccine Quality Illustrated is now available for free download and online use
read more
EPELA authentic e- Pharmaceutical Cold Chain Management course receives 2015 Hermes Creative Award as Gold Winner in e-learning category
On 1 May 2015, Hermes Creative Awards announced… read more